|
There is no one-stop, comprehensive database at this time that crosses all sporthorse breeds, disciplines, countries, etc. With the crossing of bloodlines across registries, you’ll need to be very resourceful and explore multiple research options to gain a more complete representation. The following resources are listed as some suggestions to get you started along with some notes as to why/how we use various sites. There are pros and cons to users being able to edit data so remember to use caution when evaluating/relying on data on sites that do allow that option.
Depending on the information you are looking for, the bloodlines, and the discipline, you'll find your favorite sites to utilize. We'd love to keep adding to this list, so send in your recommendations or helpful hints to [email protected].
3 Comments
12/13/2017 The Major Causes of Damage to Sperm During Freezing…water and salts and ice, oh my!Read Now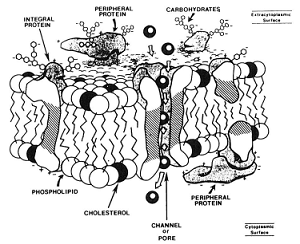 Courtesy of Paul Loomis, Select Breeders Services I have always been fascinated by the exquisite design of biological systems. The more we humans understand about biology, the more we realize we don’t know. The process of mammalian fertilization is one of these complex biological systems that in nature requires the proper coordination of so many factors ranging from the behavior of male and female to biochemical changes at the cellular and molecular level. Defined as: “A process in sexual reproduction that involves the union of male (sperm) and female (ovum) gametes (each with a single, haploid set of chromosomes) to produce a diploid zygote”, fertilization requires that functionally viable sperm, at the right stage of maturity, are present in the oviduct of the mare during a brief window of time when a functionally viable oocyte is present. Spermatogenesis Spermatogenesis (the process of sperm formation in the testes) is an ongoing process with newly formed sperm being produced constantly during the sexually mature lifespan of the male. In the stallion, spermatogenesis takes approximately 57 days from start to finish. Further sperm maturation occurs during transit of the sperm through the epididymis where motility and fertilizing capacity is acquired. Mature sperm are then stored in the tail of the epididymis and the ampulla until ejaculation. At ejaculation, the sperm are exposed to factors in fluid from the accessory sex glands that are important in protecting the sperm from the immune system of the mare. Once ejaculated into the mare’s uterus the sperm undergo further changes in response to specific signals from the female reproductive tract. These physical and biochemical changes (capacitation and the acrosome reaction) are required for the sperm to bind to and penetrate the oocyte and initiate fertilization. Once the changes associated with capacitation and the acrosome reaction occur, the sperm have a finite period to encounter a mature oocyte after which they rapidly die and are no longer able to participate in fertilization. So, successful fertilization requires that there are sufficient “functionally viable sperm” that have survived the transit through the female reproductive system, that are at the right stage of maturation at the right time and in the right place (oviduct) when a mature oocyte is present. Prolonging the Lifespan of Sperm The use of artificial insemination (AI) with cooled and frozen semen is our attempt to remove some of the natural barriers to successful fertilization such as geographical distance between sire and dam or asynchrony of optimum time for mating. AI is also used to improve efficiency of breeding by allowing for multiple mares to be inseminated from a single ejaculate. AI with frozen semen allows for International distribution of superior genetics and the possibility to access sperm from exceptional sires that are no longer fertile or even deceased. To prolong the lifespan of sperm after ejaculation and interrupt the normal timing of sperm maturation and death, semen processing laboratories will slow down or completely halt metabolism by cooling or freezing the sperm. Without proper processing, cooling and/or freezing of sperm causes lethal damage to the cells. In this article, we will discuss the major causes of sperm damage during cryopreservation and what steps are taken to minimize that damage. A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. For example, in a saline solution, salt is the solute dissolved in water as the solvent. Ejaculated semen consists of spermatozoa in a suspension of seminal fluids. In an isosmotic (or isotonic) physiological state, the concentration of solutes dissolved in the seminal fluid is equal to the concentration of solutes within the cell. The membranes that surround the spermatozoa can allow for the movement of water and ions across the cell membrane to maintain this equilibrium and keep the osmotic pressure equal across the cell membrane. In a suspension of cells, a solution that contains a concentration of solutes higher than that inside of the cell is hypertonic and one that has a lower concentration of solutes is hypotonic. The membranes that surround compartments of the sperm are comprised of a bilayer of various lipids and proteins (diagram above left) that are arranged to serve certain functions including transport of molecules, ions and water between the inside and outside of the cells. At body temperature, these lipid membranes are fluid and the proteins can move laterally within the membrane. At reduced temperatures, the lipids undergo phase changes from liquid to gel to solid states and the arrangement of membrane components can be altered, leading to changes in permeability, premature capacitation and eventual cell death. Sperm Damage During Cryopreservation The traditional theory of sperm damage during cryopreservation is the two-factor hypothesis. In this theory, the two major factors contributing to damage are:
Conversely, if the cooling is too rapid then the water doesn’t have time to move outside the cell and large intracellular ice crystals form which can physically damage the cells. The ideal cooling curve (right) to minimize cell damage is one which is slow enough to allow for partial dehydration of the cell and avoid large intracellular ice crystal formation but fast enough to minimize damage from solution effects. To minimize damage, sperm are suspended in extenders that contain a number of components designed to protect the sperm during cooling and freezing. In addition to salts, buffers, water and antibiotics both non-penetrating (sugars) and penetrating (glycerol, DMSO, ethylene glycol, amides) cryoprotective agents (CPA’s) are included. Freezing extenders also typically include sources of lipid and lipoprotein from egg yolk and milk. The low-density lipoproteins in egg yolk and the casein in milk are thought to protect the sperm from cooling damage and may play a role in membrane repair. Penetrating CPA’s like glycerol, ethylene glycol or the amides protect sperm by lowering the temperature at which the cells are exposed to critically high salt concentrations while non-penetrating CPA’s like lactose protect the cells through osmotic properties that promote rapid dehydration of cells. However, evidence from recent studies with human and horse sperm indicate that with the cooling rates typically used for cryopreservation of sperm there is little or no formation of intracellular ice. Therefore, the major source of damage may be primarily due to osmotic stress caused by extracellular ice formation and the resulting changes in relative cell volume during freezing and thawing. Let’s consider the changes in cell volume (see graph below) that likely occur during a typical freeze-thaw cycle. For horse semen cryopreservation protocols:
If the volume change exceeds the osmotic tolerance limits of the membranes then they will be irreversibly damaged and cause cell death. Species differ in the susceptibility of their sperm to damage due to cold shock and cryopreservation. These species-specific differences are thought to be related to the biochemical structure of the plasma membranes, specifically the cholesterol:phospholipid ratios, fatty acid content and membrane fluidity. It is believed that these differences are likely responsible for the differences in osmotic stress tolerance seen between species whose sperm survive cryopreservation well versus those that do not. Male to Male Variation Within a Species In addition to this species-specific variability, a well-documented inherent variation exists between individual males of many species in the ability of their sperm to withstand the stresses associated with freezing and thawing (cryotolerance). This male to male variation is especially evident in stallions. In dairy cattle, bulls have been selected by the AI industry for more than 50 years based on the ability of their sperm to withstand the stresses of standard cryopreservation protocols. This selection has led to an increasingly uniform and positive response to cryopreservation. Studies on membrane fluidity and osmotic stress tolerance have demonstrated that bull sperm have a much greater tolerance for exposure to hypertonic conditions than stallion sperm and that there was a 3-fold greater variance in osmotic stress tolerance between individual stallions than between individual bulls. Studies with boar sperm and human sperm have also revealed significant male to male variation in plasma membrane composition and some correlations have been found between cholesterol to phospholipid ratios, membrane fluidity, fatty acid content and response to cryopreservation. Further evidence for the relationship between membrane composition and cryosurvival comes from experiments with 4 different strains of mouse sperm that vary significantly in their cholesterol:phospholipid ratio. The percentage of motile sperm after thawing was directly correlated with the cholesterol:phospholipid ratio. The researchers were also able to dramatically improve cryosurvival in the low cholesterol strain by increasing the cholesterol content of the sperm membranes with cholesterol loaded cyclodextrins. The SBS Difference To date there is no single universal cryopreservation protocol that is optimum for semen from all stallions and use of a single protocol (extender, cooling rate, etc.) has led to the belief that stallions can be grouped into “good” and “bad” freezers based on post-thaw evaluation of semen frozen using a single common protocol. The SBS System for freezing stallion semen is based on the belief that semen from a large percentage of stallions in the population can be frozen successfully if an effort is made to customize cryopreservation protocols to identify optimum conditions for each individual stallion. Our goals are to:
Excluding a champion performance stallion from a commercial frozen semen breeding program based on results from a single cryopreservation protocol is not acceptable if frozen semen is to be a significant tool in modern horse breeding. The SBS approach employs multiple protocols that are designed to determine the optimum procedure for maximum fertility of frozen semen from each individual stallion. Standard practice for all new stallions is to perform 1 or 2 split-ejaculate test freeze procedures using a variety of extenders that contain different sources and amounts of lipids, proteins, sugars and various penetrating and non-penetrating cryoprotectants designed to control damaging cell volume excursions during freezing and thawing. Conclusion A great deal of progress has been made in the fundamental understanding of how sperm from many species are compromised during cryopreservation and this information has led to development of new extenders and protocols that have improved the results of frozen semen inseminations. Today, with improved freezing protocols leading to better quality frozen semen and a greater understanding of how to manage mares for insemination with frozen semen, this technology is more widespread than ever in horse breeding. And given the stress that we humans have placed upon nature’s exquisitely developed system of reproduction, one can marvel at how successful this technology has been so far. Courtesy of Select Breeders Services
Breeding mares on their foal heat is a strategy used to maximize reproductive efficiency. Since income is generated from selling offspring, yearly foal production is critical to offset maintenance and breeding expenses incurred by the mare owner. With an average gestational length of 333 to 345 days, mares must become pregnant within one month post partum to continue producing foals each year. Mating mares on the first postpartum estrus is one method used to improve the chance of maintaining yearly foal production. Reviewing this topic for us is guest writer, Dr. Margo Macpherson with an excerpt from the chapter Breeding Mares on Foal Heat co-authored by Dr. Margo Macpherson and Dr. Terry Blanchard in the 2nd Edition of Equine Reproduction. The first post partum estrus generally begins 5-12 days after foaling; hence, the terminology foal-heat or “9 day heat.” In a large study involving Thoroughbred mares, ovulation during foal heat was reported on average at 10 days post partum, with most mares ovulating within 20 days post partum (Loy, 1980). Mares that foaled early in the year were less likely to ovulate by 10 days post partum than mares foaling later in the spring. Also, early foaling mares (January to March) were more likely to have a prolonged period of postpartum anestrus (> 30 days to first ovulation postpartum) than late foaling mares. While not proven, it is suspected that the postpartum anestrus seen in many foaling mares is thus related to length of days when parturition occurs. In this regard, supplementing artificial light to late pregnant mares has been shown to reduce the incidence of postpartum anestrus (Palmer and Driancourt 1983). Advantages for mating on foal heat include:
Uterine Repair In the normal foaling mare, several events occur to the uterus in the immediate post-foaling period. This is termed uterine involution. Inflammation helps rid the uterus of debris and contaminants that are present in the uterus for the week after foaling. This process results in the formation of fluid, called lochia, which is often seen coming from the mare’s vulva. This fluid is usually reddish-brown and does not have a foul odor. The uterus, which is a strong muscle, actively contracts after foaling to help evacuate the fluid and debris. Contractions are also important for returning the uterus to its pre-foaling size. Suckling by the foal and exercise are important stimulants for uterine contractions and evacuation. Tissue repair and uterine “clean up” are essential for re-establishing pregnancy. Involution occurs rapidly after a normal parturition. Within 30 days of foaling, the uterus should have returned to its’ pre-foaling state in both tissue health and size. Major factors thought to be related to fertility achieved on foal heat breeding include an uncomplicated birth free from genital tract trauma, prompt passage of the placenta, rapid uterine repair/involution, and early return to regular estrous cycles. Selection of mares for foal heat breeding should be based on meeting these minimum criteria to optimize success. Strategies For Breeding Mares on Foal Heat Breeding mares on foal heat is not a recipe-driven event. Each mare must be treated as an individual and the conditions of her foaling considered. In general, young mares with uncomplicated foaling are better candidates for breeding on foal heat than older mares that have previously delivered several foals. A general approach to breeding mares on foal heat starts in the week after foaling. First, all mares should be examined no later than 6-8 days after foaling. A visual examination of the mare’s reproductive tract will reveal the presence of urine pooling, pneumovagina or unresolved trauma to the vagina, vestibule or vulva. Although these conditions usually will improve over time, injured mares are not good candidates for breeding on the first postpartum estrus. Examination of the internal reproductive tract using an ultrasound should be performed to reveal the presence of intrauterine fluid accumulation and size of any developing follicle(s). These factors are monitored by repeating examinations at 1-2 day intervals. If fluid is present in the uterus at the time when foal heat breeding is anticipated, it is better to treat the mare to remove the fluid than to breed the mare. Mares judged to be involuting normally, with no significant intrauterine fluid accumulation, are good candidates for foal heat breeding. If the mare ovulates prior to day 10 post partum, breeding on foal heat can be bypassed and prostaglandin can be administered 5-6 days after ovulation to induce an earlier return to estrus for breeding. It is thought that this protocol allows the uterine environment more time for repair, as well as provides a shorter interval (1-2 weeks) to breeding than that achieved by waiting for the second postpartum estrus to spontaneously occur (typically around day 30). Some mares will accumulate fluid after the foal heat breeding. Such mares should be treated to remove fluid and resolve any infection that may have been established. Performing uterine lavage (3-5L lactated ringers or saline solution) in these mares is important, and is thought to not interfere with fertility if the lavage is performed at least 4 hours after breeding (Brinsko et al 1991). Use of drugs to promote uterine contraction, such as oxytocin, can also be helpful when treating mares with uterine fluid. Often, oxytocin is combined with uterine lavage for fluid removal in the breeding period. While the inclusion of antibiotic infusions after breeding postpartum mares remains controversial, some individuals (Pycock, 1994) have reported that postpartum mares treated with antibiotics plus oxytocin had higher pregnancy rates than either mares treated only with oxytocin or mares that were left untreated. In conclusion, not all mares are suitable candidates for breeding during the first postpartum estrus. However, using careful selection of mares, breeding during foal heat can result in favorable pregnancy rates in a highly efficient manner, and can reduce parturition to conception interval to help to maintain yearly foal production in mares. Dr. Margo Macpherson received her DVM degree in 1990 from Michigan State University after which she completed a residency and Master’s Degree in Equine Theriogenology at Texas A&M University. After leaving Texas, Dr. Macpherson spent time at the University of Pennsylvania and in private practice in Central Kentucky. Currently an Associate Professor and Service Chief in the section of Reproduction at the University of Florida, Dr. Macpherson is primarily interested in conditions that affect pregnancy including twin pregnancy and placentitis. References Blanchard, T.L. and Macperson M.L. 2011. Breeding Mares on Foal heat. Equine Reproduction, 2nd Edition, Editors: A.O. McKinnon, E.L. Squires, W.E. Vaala and D.D. Varner, Wiley-Blackwell, West Sussex, UK. Brinsko, S.P., Varner, D.D., Blanchard, T.L. 1991. The effect of uterine lavage performed four hours post insemination on pregnancy rates in mares. Theriogenology 35:1111-1119. Loy, R.G. 1980. Characteristics of post partum reproduction in the mare. Vet Clin N Amer: Large Anim Prac 2:345-359. Palmer, E., Driancourt, M.A. 1983. Some interactions of season of foaling, photoperiod and ovarian activity in the equine. Livest Prod Sci 110:197-210. Pycock, J. 1994. Assessment of oxytocin and intrauterine antibiotics on intrauterine fluid and pregnancy rates in mares. Proc Amer Assoc Eq Pract 40:19-20. Natalie DiBerardinis
Breeding Manager, Hilltop Farm So one of the important topics that has been under discussion over the past ten years or so is this: Do the Young Horse classes really predict talent for the future FEI horse or are they rewarding extravagent movers that won't be able to handle more collected work that is the core of the FEI work? It's a complicated question, with many factors including pre-selection, training, etc but overall, I do believe we see a far greater number of these horses progress into the FEI levels. I reviewed the USEF National Championships from 2011-2016 for the top 5 placing horses in the FEI 5-year old & FEI 6-year old classes to see what their own development has shown us. That gave me 51 horses to look at (some horses placed in the top 5 both years, and I didn't look at the 2016 5-year olds as they aren't old enough to compete in the FEI classes). Of those 51 horses, 30 are competing at Prix St. Georges or above. That's a 'graduation rate' to the FEI ring of almost 59% (there are also 2 horses competing in the FEI 7-yr old Division but I didn't count them in my statistics). Nine of these horses have competed at least to the Developing Grand Prix level, and one was a member of the Pan-American Gold Medal Team. Keep in mind also that these horses currently range from ages 7 to 12. To add another layer, I also looked at how many of these horses were US-bred (Full disclosure: I did include one by a US-breeder who bred this particular horse in Germany). Fifteen of the 30 horses now competing at the FEI levels are US-bred. Those are some very strong stats for the top 5 finishers! **US-Bred**Somer Hit (Sandro Hit-Rotspon), breeder Mo Swanson Lauren Chumley/Alice Tarjan 3rd in 2011 (5yr) with 8.408/1st in 2012 (6yr) with 8.050 Showing Int II-Grand Prix by age 8 Sanceo (San Remo-Ramiros Son II) Sabine Schut-Kery 4th in 2011 (5yr) with 8.116 Team Gold Medal at 2015 Pan-Am Games, Showing Grand Prix Elfenfeuer (Florencio I-Sion) Alice Tarjan 2nd in 2012 (5yr) with 7.864; 5th in 2013 (6yr old) with 7.832 Showing Int II-Grand Prix by age 7 **US-Bred**Freedom (Feuri x Windjammer), breeder Barbara Cadwell Patrica Becker 3rd in 2012 (5yr) with 7.612 Showing Grand Prix Horizon (Hotline x Revue) George Williams 4th in 2012 (5yr) with 7.592 Showing PSG/Int I with Adrienne Lyle Fashion Designer OLD (Faustinus x Forst-Design) Nadine Buberl 1st in 2013 (5yr) with 9.012; 3rd in 2014 (6yr) with 8.12 Showing Inter II with Cesar Parra Fiderhit OLD (Fidertanz x Fleur) Nadine Buberl/Cesar Parra 3rd in 2013 (5yr) with 8.620; 6th in 2012 (6yr) with 7.798 Showing in FEI Young Riders with Barbara Davis **US-Bred**Qredit Hilltop (Quaterback-Dream of Glory), breeder Judy Yancey Christopher Hickey 4th in 2013 (5yr) with 8.480 Shown through Intermediate I with Michael Bragdell **US-Bred**Benefactor RRS (Bonheur-Rubinstein), breeders Melinda Walton & Larry Smith Silva Martin 5th in 2013 (5yr) with 8.452 Showing PSG Emilion SA (Painted Black x Karisa) Kelly Casey 1st in 2014 (5yr) with 8.680 Shown to PSG Elian (Sir Oldenburg x Barliane) Anna Marek 3rd in 2014 (5yr) with 8.14 Showing PSG **US-Bred**Wakeup (Wagnis-Macho), breeder Beverly McLean Tetrick Emily Wagner 1st in 2011 (6yr) with 8.736 (also won as a 5yr old) Competed at 2010 World Young Horse Championships, won Developing PSG Championships & 2nd at Developing GP Championships, Showing Grand Prix **US Breeder**Bon Chance (Belissimo M-Weltmeyer), breeder Marefield Meadows Caroline Roffman 2nd in 2011 (6yr) with 8.736 Shown to Grand Prix, now in U25 Aesthete (Trento B x Unusual) Silva Martin 3rd in 2011 (6yr) with 8.728 Now showing Inter II Riccidoff (Riccione x Don Sarina) George Williams 4th in 2011 (6yr) with 8.084 Showing PSG/Int I with Adrienne Lyle Adje (Rousseau-Jazz) Willy Arts 5th in 2011 (6yr) with 8.076 Showing PSG Simply Nymphenburg (Sir Donnerhall I x Wendy) Cesar Parra 2nd in 2012 (6yr) with 7.796 Showing in FEI Young Riders with Barbara Davis Vitalis (Vivaldi-D-Day) Charlotte Jorst 1st in 2013 (6yr) with 9.152 Showing PSG/Int 1 with Isabel Freese Sunshine Tour (Sir Donnerhall x History) Jane Karol 2nd in 2013 (6yr) with 8.028 Winner of 2016 Developing GP Championships, showing GP **US-Bred**Caliente DG (OO Seven x Satina), breeder DG Bar Ranch Craig Stanley 3rd in 2013 (6yr) with 7.940 Showing PSG/Int I **US-Bred**Clapton JP (UB40 x Liana), breeder Peggy Mills Holger Becktloff 4th in 2013 (6yr) with 7.782 Showing Grand Prix **US-Bred**Ripline (Hotline x Riviera), breeder Oak Hill Ranch Heather Blitz 1st in 2014 (6yr) with 8.612 Showing PSG/Int I **US-Bred**Hemmingway (Hofrat-Archipel), breeder Angela Barilar Angela Jackson 2nd in 2014 (6yr) with 8.572 Showing PSG/Int I **US-Bred**Sir Steinerman (Stedinger x Donnabella), breeder Marlace Hughes Amy Lewis 4th in 2014 (6yr) with 7.994 Showing PSG **US-Bred**Doctor Wendell MF (Don Principe-Sandro Hit), breeder Marydell Farm James Koford 5th in 2014 (6yr) with 7.976 Showing Int II/Developing GP, winner of 2015 US Dressage Finals at PSG **US-Bred**Gallant Reflection HU (Galant du Serein-Rohdiamant), breeder Horses Unlimited Lisa Wilcox 1st in 2015 (6yr) with 8.136 Showing Int II **US-Bred**Floretienne (Florestan-Jazz), breeder Judy Yancey Emily Wagner Miles 2nd in 2015 (6yr) with 7.836 Showing PSG/Int I Ellert HB (Johnson x Alanda-B) Jordan Rich 4th in 2015 (6yr) with 7.676 Showing PSG Lucky Strike (Lord Laurie x Heidi) Endel Ots 1st in 2016 (6yr) with 8.604 Competed at 2015 & 2016 World Young Horse Championships, showing Developing PSG **US-Bred**Sternlicht Hilltop (Soliman de Hus-Rascalino), breeder Rachel Ehrlich Michael Bragdell 3rd in 2016 (6yr) with 8.028 Competing PSG Susanne Manz, Manz Dressage Horses
Twin Cities area, Minnesota I attended the KWPN – North America Annual General Meeting in Lexington, Kentucky last week. It was a fun and educational event, offering the chance to meet and network with other breeders. It’s always fun to hear what other breeders are doing and to share experiences with them. Some of the more experienced breeders shared their experiences during a panel discussion. Bert Rutten from the Netherlands was a special guest and provided insights on selecting, breeding, and developing dressage horses. As a member of the 1984 Olympic team and current head of the KWPN Stallion Selection Committee, Bert had excellent perspectives on dressage horse breeding and development at all levels. Bart Henstra from the Netherlands also attended and gave a clinic on linear scoring of KWPN horses as well as a presentation on the new OCD genome testing that the KWPN is doing. Dr. Scott Harper from Rood and Riddle discussed pre-purchase examinations and Dr. Debbie Harrison gave an update on the latest breeding information form AAEP. We had a field trip to Spy Coast Farm where we watched horses in the jumping chute. Then on to the Kentucky Horse Park where we had some time to wander through the Horse Museum and visit the KWPN–NA office. On the second day we had a field trip to Valley View Farms. Willy Arts coached the Young KWPN–NA members on in-hand handling and presentation. It was fun to watch but I was glad I did not have to run the horses! We also had fun getting to see a harness horse presentation and discussion from Wim Cazemier and Sterling Graburn. Wim showed us his beautiful harness stallion, Colonist. Thanks to the presenters but also to the owners of the beautiful demonstration horses! It takes a team effort to put together such an educational meeting. Having demonstration horses available makes a huge difference. It’s nice to see how other breeders share their knowledge and contribute resources to newer breeders like me. It was a warm, supportive environment. The KWPN–NA Board of Directors did an awesome job. A couple of interesting points –
So, to my USSHBA friends and colleagues, meet the KWPN North American Association. And KWPN friends, please meet the USSHBA. Please visit the USSHBA booth at the FEI World Cup in Omaha at the end of March. |
Details
AuthorsA collaborative effort produced by the USSHBA Education Committee, USSHBA members, and our partners. Archives
January 2021
|
